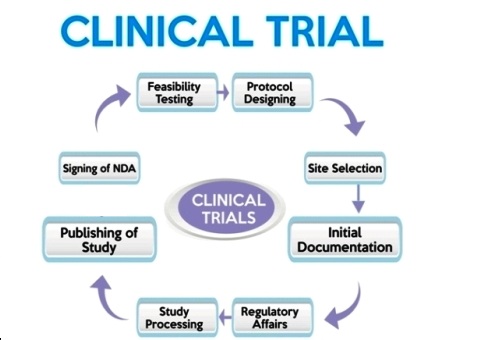
Clinical Trials
Clinical trial involves the drug testing or research on human subjects or volunteers. It is an investigation or series of investigations comprising of administration of drug product to human subjects so as to obtain the efficacy and safety proof that the drug have certain therapeutic effects which are beneficial to the patients administrating it. Approval is taken from Ethical Committee for the conduction of experiments on human. It comprises of different phases:
Phase 0 or Micro-dosing Phase - This phase involves the administration of very small dose of the drug under study so as to check any unwanted effects , if occur then further preclinical trials are done to confirm the safety.
Phase 1 - This phase carries Experimental Intervention ( in this two groups are made, to one group tested drug is administered and to other group placebo is administered) .It requires lesser number of subjects i.e. 20 and may include healthy volunteers. This phase evaluates and determines the pharmacokinetics, pharmacodynamics and toxic behaviour of the drug under investigation.
Phase 2 - This phase involves dose- response relationship, determination of Effective dose and drug safety issues. In this phase hundreds of subjects are considered who are suffering from the disease for which drug is under study.
Phase 3 or Confirmatory Phase - This phase requires large number of subjects and helps in confirming the drug efficacy. This phase involves registration of the drug. Comparison of the study drug with the existed drug is done in the subjects.
Phase 4 or Post Marketing Surveillance - This phase starts after the drug entered in the market. This phase is longest and is done to check the drug safety in large population. This phase helps in determining the long term safety of the drug under study.