Drug Development
NOTE: We help in the herbal and Nutraceutical formulation making specific to a diseases or cause.
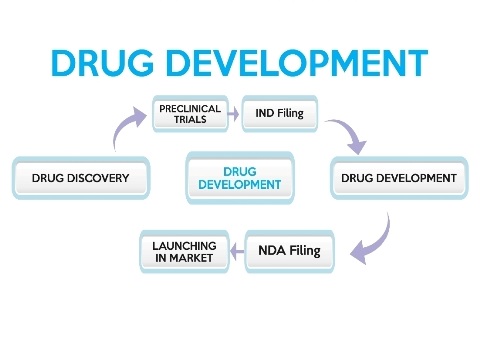
Generally Drug Development involves the identification of most promising compound i.e. showing some therapeutic effect. This can be either done by modification in the existing compound or developing a novel compound.
After this the compound is tested on the animals to check the safety profile of the compound. In this minimum effective dose and minimum lethal doses are assessed. The evaluation is done that whether the compound is genotoxic, teratogenic, mutagenic, carcinogenic or causing reproductive impairment.
If the drug is found safe in animal study then Investigational New Drug is filed so as to take approval for running of human or clinical trials.
Various stages of human trials are conducted to check the safety and efficacy of the drug in human volunteer. After this New Drug Application is filed for launching the drug in the market